Collagen is one of the most popular supplements today. It is believed to promote better cartilage, tendon, and joint health, which is crucial for athletes as it may lower the risk of injuries and enhance recovery after connective tissue injuries. Hence, it is worth looking at the mechanism and overall scientific evidence of its effectiveness on pain reduction and joint health.
Background
In 2019, it was estimated that 57 million people in Western Europe had osteoarthritis, a condition in which the articular cartilage wears away, exposing the underlying bone, often causing chronic pain and requiring surgical treatment (1). Among athletes, connective tissue injuries are the most commonly reported types of injuries. As a result, there is significant interest in finding strategies that can improve the quality of soft tissues and enhance the healing properties of cartilage, tendons, and ligaments.
In this context, collagen is of major interest, as it provides structural support to the extracellular space of connective tissues. Collagen is the most abundant protein in connective tissue, particularly type I collagen. The strength and stiffness of our connective tissues and tendons are determined, in part, by the amount of collagen they contain. It is reasonable to assume that improving collagen synthesis may indirectly lead to better joint and connective tissue quality and decrease the risk of injuries (2).
In this article, you’ll find a summary of the current evidence regarding the effects of collagen supplements on improving cartilage and tendon health
Collagen is a protein structure composed of amino acids. Unlike muscle tissue, it contains fewer essential amino acids but more proline and glycine.
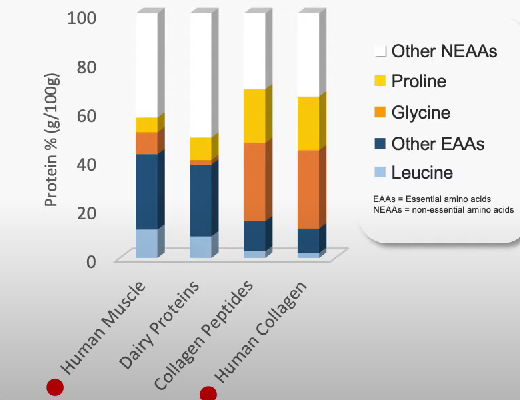
This suggests that consuming certain nutrients rich in proline and glycine may stimulate collagen synthesis and enhance joint health for athletes and individuals with arthritis. Two key dietary protein sources in this context are gelatin and collagen hydrolysate, as they contain higher levels of proline and glycine compared to other protein sources.
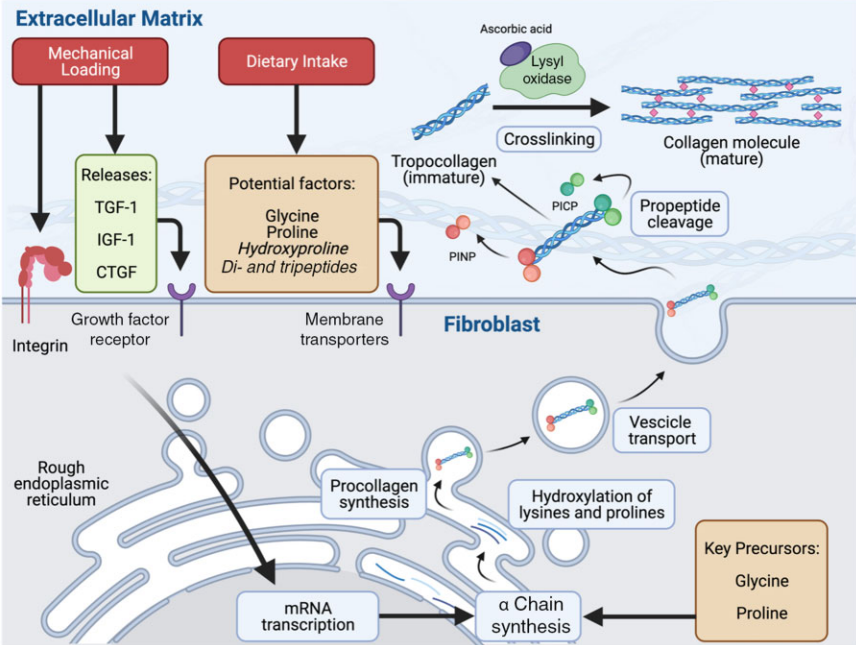
Collagen metabolism (3)
Isn’t gelatin and collagen considered to be of low protein quality?
Gelatine and collagen have a relatively low protein quality, particularly in terms of their biological value. This biological value assesses the amino acid composition of protein-rich foods compared to that of human muscle. (4). Recent studies have shown no positive effect of collagen supplementation on muscle protein synthesis (5). So yes, gelatine and collagen are not well-suited candidates to make you look jacked. However, this does not mean they cannot positively impact other tissues.
How well is gelatine digested and absorbed?
A mechanistic study conducted in 1999 found that gelatin hydrolysate is absorbed by mice, leading to its accumulation in cartilage tissue.(6).
Nearly two decades later, researchers verified these findings in humans. (7). In a crossover study, eight healthy subjects consumed either 0, 5, or 15 grams of gelatin enriched with 48 mg of Vitamin C 1-hour before completing 6 minutes of rope skipping.
The findings:
Consuming 15 grams of vitamin C-enriched gelatin led to a significant increase in the blood concentrations of glycine and proline. This suggests that these “lower quality” proteins are effectively absorbed and substantially raise the levels of these amino acids, which are essential for collagen synthesis.
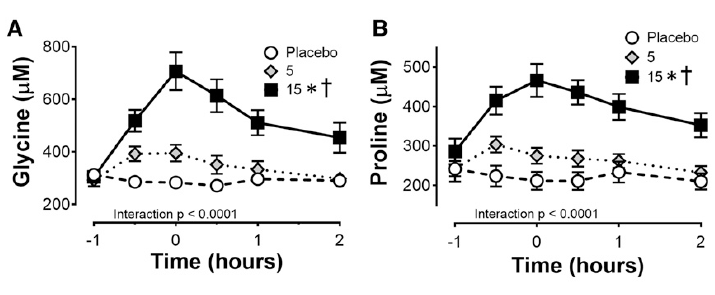
Glycine and proline concentrations in the blood after consuming 0, 5 or 15g of Gelatine – Vitamin-C enriched (7)
Moreover, a notable increase of 153% in the collagen synthesis rate was observed, as measured by the levels of amino-terminal propeptide (PINP), which is a precursor to collagen that includes a short signal sequence and terminal extension peptides. The researchers also noted a higher concentration of collagen in the engineered ligament.
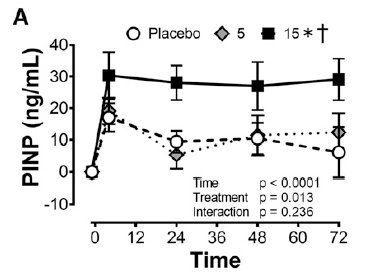
Collagen synthesis rate measured via PINP, with 0, 5 and 15g of Gelatine – Vitamin-C enriched (7)
What about collagen?
The same group of researchers did a follow-up study, in which they compared the effect of 15g of hydrolysed collagen vs. 15g gelatine (vitamin-c enriched) vs. gummi bears that contained both gelatine and collagen (8).
This is what they found:
The results showed that both – collagen supplementation and gelatine + vitamin-C resulted in an approximate 20% increase in the collagen synthesis rate. However, this change was not statistically significant.
When looking closely at the individual response of the participants, a significant variance becomes evident. Further analysis revealed that the overall positive effect may have been driven by one positive outlier in both the gelatine and collagen groups. Notably, in the gelatin group, three out of eight participants showed no positive change in collagen synthesis, while four out of eight exhibited no effect in the collagen group.

Thus, it is uncertain whether these positive findings can be reliably applied to the general public.
It is important to note that the researcher again utilized a manufactured ligament in both studies. Up to this point, we still lack human studies that confirm these findings, which is understandable given the difficulty in obtaining a sample of human cartilage tissue.
Recent studies have reported mixed results. Two studies (9, 10) observed increased collagen synthesis with collagen supplementation compared to a placebo, whereas another study (11) found no effect. The reason for the lack of effect in the latter study is unclear to me, as all three studies were conducted under very similar conditions. I suppose we’ll need to wait for more data before we can make a more accurate assessment of its effectiveness
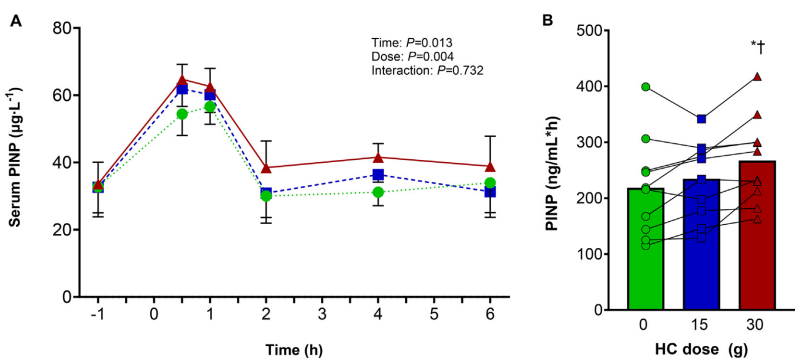
Indirect measurement of collagen synthesis via serum PINP levels (10)
Moreover, it should be considered that collagen synthesis rates were measured indirectly through blood markers. While the N-terminal propeptide of type 1 pro-collagen (PINP) is indeed an indirect marker for type 1 collagen, it does not indicate where this synthesis occurs. Type 1 collagen is the most abundant type of collagen in the body, found not only in cartilage and ligament tissues, but also in bones, skin, and muscle.
This does not allow for conclusions to what happens in the connective tissue itself.
MRI images
The following study is exceptionally well done and doesn’t have these limitations:
McLindon and colleagues (11) investigated the effects of daily supplementation with 10g of collagen hydrolysate on mild knee osteoarthritis over a duration of 48 weeks. The extended duration of the study is certainly a significant advantage. The researchers also evaluated cartilage function using dGEMRIC imaging, which serves as a more reliable indicator of changes in cartilage tissue than measuring its acute synthesis rate over shorter periods (12, 13).
And indeed, a positive effect can clearly be seen when looking at the images below: The lower photo series is from the collagen supplementation group and shows more green = better cartilage health.
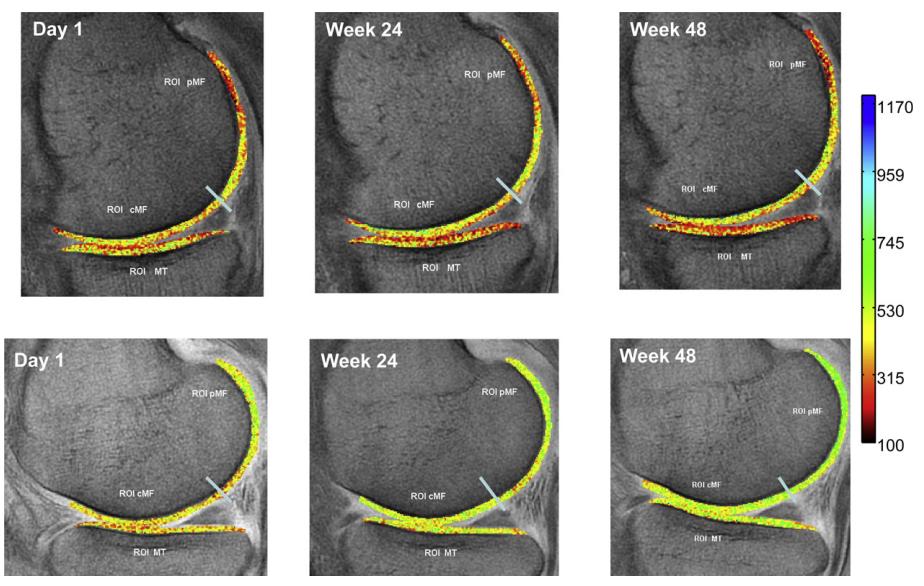
lower photo series is from the collagen supplementation group and shows more green = better cartilage health
Does that prove that collagen supplementation is indeed effective for improving cartilage damage?
Not so fast!
The dGEMRIC images showed improvements of cartilage in the posterior lateral femur region, with the collagen group demonstrating statistically better improvement than the control group.
Further MRI images of cartilage tissue in other regions (such as the medial tibia, central medial femur, posterior medial femur, lateral tibia, and central lateral femur) did not reveal any differences between the collagen and placebo groups.
The author’s conclusion was:
[…] because of the pilot nature of this study, and its small sample size, we do not regard these results to be definitive, and the CH intervention merits further testing in a larger study.
It is unclear why the collagen group demonstrated improvements in some areas but showed no changes in others. The small sample size of only 15 participants per group makes it difficult to draw definitive conclusions. Unfortunately, there are no other studies using dGEMRIC images to evaluate changes in cartilage structure
Effect of collagen on tendon and ligament stiffness & functionality
The same study mentioned above (11) also assessed potential changes in physical functionality and stiffness but failed to find a positive effect of collagen supplementation. It is important to note that participants did not adhere to a systematic training regimen designed to strengthen muscles, tendons, or other soft tissues. This lack of structured resistance exercise may explain why McAlindon et al. (2011) (11) did not observe a positive effect, as resistance training is a necessary stimulus for collagen synthesis (14).
In a study conducted by Leet et al. (2003), it was found that female football players who received daily collagen hydrolysate supplementation (30g, along with 50mg of vitamin C) experienced a modest improvement in patellar tendon stiffness. The results showed that the collagen group had an increase of 18.0 ± 12.2% (effect size d = 1.11) compared to the placebo group, which had an increase of 5.1 ± 10.4% (effect size d = 0.23), with a significant difference noted (p = 0.049). However, the growth of the tendon was similar between both groups. (15).
In another study, 40 healthy young men trained their calf muscle and Achilles tendon for 14 weeks, 3 training sessions per week, and took 5g of collagen peptides (CP – Temdeforte®) (16). As far as I know, this is the only study in which participants followed a structured resistance training protocol for more than a 3-months period while progressively increasing training weight. This may explain why the authors detected a significantly (p = 0.002) greater increase in tendon growth (CSA +11.0%) compared to the PLA group (+4.7%). In contrast, there was no difference in tendon stiffness or muscle strength increase between groups.
In contrast to these, are the findings of (17), who found that collagen supplementation does indeed tending growth. To my knowledge, this is the only study that implemented a structured resistance training program for more than three months while progressively increasing the training weights. This approach may explain why the authors observed a significantly greater increase in tendon growth (cross-sectional area increase of 11.0% in CS vs. 4.7% in PL group; p = 0.002). There were no significant differences in tendon stiffness or muscle strength increase between the two groups.
Faster recovery?
Praes et al. (2019) (18) studied 10 participants with Achilles tendinopathy, administering 2.5 grams of collagen peptide daily for six months along with a bi-daily calf-strengthening program. The results showed no significant benefits on tendon microvascularity, ankle functionality, or pain. The authors did find a positive trend towards faster return to sport was observed in athletes who took CP. However, the small sample size made it impossible to conduct a thorough statistical analysis. This raises the question of whether the observed differences were merely due to chance rather than actual effects of the supplementation.
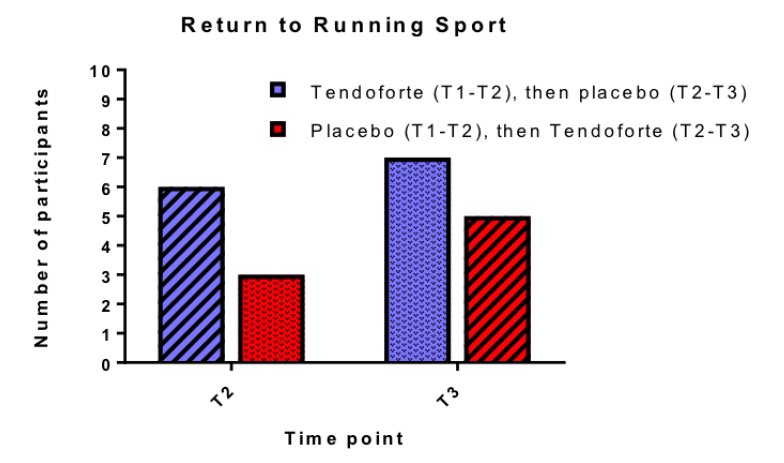
Praet et al., (2019): due to low number of participants, researchers did not carry out a chi-square t-test
Interestingly, this study is often referenced in discussions regarding the effectiveness of CP for athletes
Can it reduce pain?
Multiple studies indicate that collagen supplements may provide a small benefit for arthritis-related pain (19,20,21). This improvement in pain sensation was measured both subjectively from the patients and confirmed by standardized tests conducted by doctors.
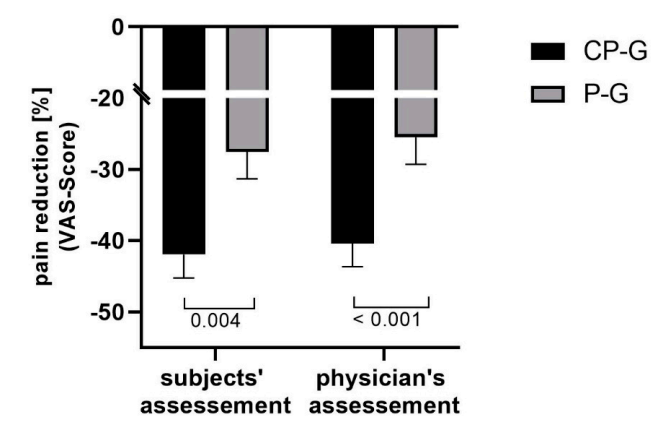
Relative changes in activity-related pain (VAS Score) (19)
However, it should be noted that other studies didn’t find a positive effect on pain reduction (22).
Pain management – practical implications
From a practical perspective, I would categorize collagen as “worth trying” for anyone experiencing arthritis-related pain. If you don’t notice any improvement after approximately 8 weeks, it’s best to discontinue its use. Generally, the overall effect is likely to be modest, as most studies indicate a reduction in pain sensation of about 15-20% compared to a placebo.
Effectiveness of Whey and Casein
It is reasonable to assume that high-quality protein sources, such as whey or casein protein, would positively impact collagen synthesis, especially since lower-quality protein sources have shown some effectiveness.
However, research indicates otherwise. Multiple studies examining whey protein found no positive effect on the rate of collagen synthesis or intramuscular connective tissue (23, 24).
One hypothesis for why these studies did not find an effect on connective tissue synthesis is that whey protein is metabolized too quickly. This rapid metabolism may lead to the activation of connective tissue occurring too late, suggesting that a slower-absorbing protein might be more effective in optimally stimulating the process.
source: Nutrition Tactics
This hypothesis was investigated by Trommelen et al., (2020) (25). They found that exercise boosts the rate of connective tissue synthesis, but consuming 30g of casein protein after resistance training did not provide any additional benefits.
Connective tissue proteins have high concentrations of proline (12%) and glycine (25%) compared to other proteins found in skeletal muscle. In contrast, casein contains only small amounts of proline (6.5%) and glycine (2%), which may be insufficient to support the post-exercise increase in muscle connective tissue protein synthesis. This hypothesis is further supported by research from Trommelen et al. (2020), which showed that administering casein did not elevate glycine concentrations in the blood (25).
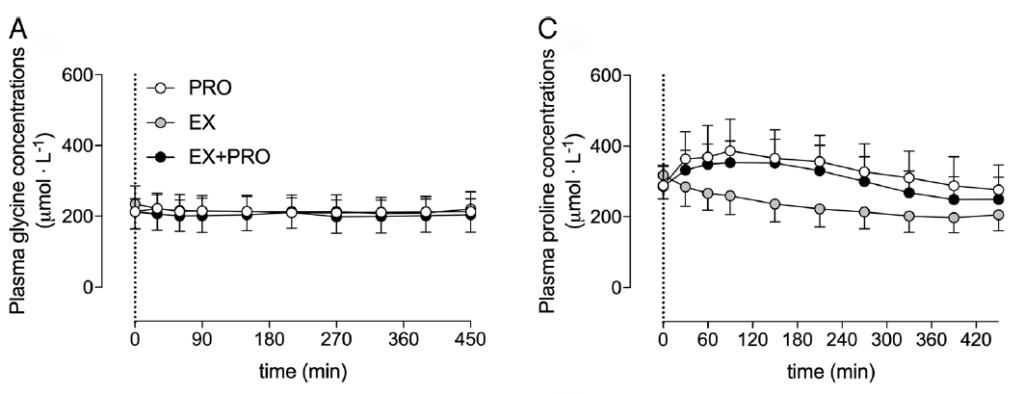
Changes in glycine and proline concentrations after consuming 30g of casein (Trommelen et al., 2020)
What if the amount of consumed protein were increased?
This question was investigated by a follow-up study of the same researchers (26): they raised the protein intake to 40g of casein protein to assess its effects on connective tissue synthesis after resistance training in older men. The findings revealed that this increased consumption actually does have a positive impact on the rate of connective tissue synthesis.
We demonstrate for the first time that dietary protein-derived amino acids are incorporated into de novo muscle connective tissue protein in older adults
However, the significance of these results is questionable because measuring connective tissue synthesis rates does not provide insights into what is happening in other connective tissues, such as ligaments or cartilage.
Considerations for female athletes
Oestrogen has the potential to influence collagen turnover in human connective tissue, as oestrogen receptors are present in tendon (27). Thus, the fluctuation of circulating oestrogen concentration may affect the mechanical properties of these tissues (28) at different stages of the menstrual cycle.
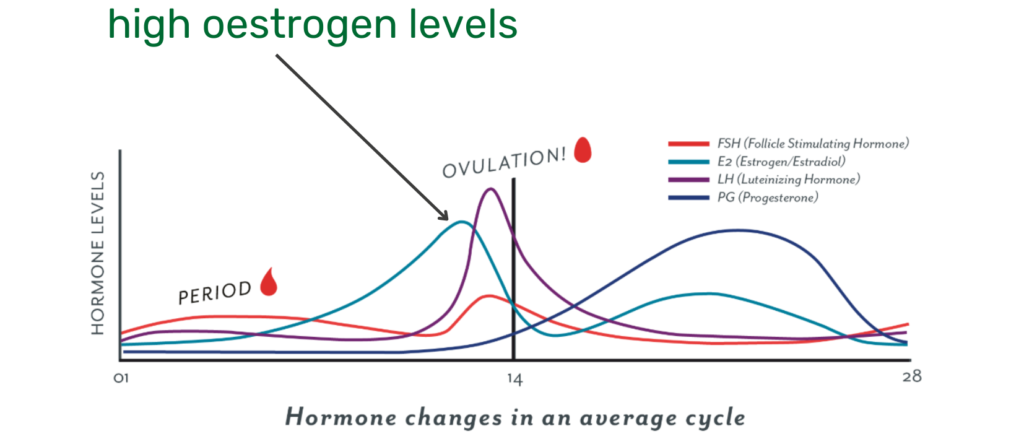
At this point, we only have a case study available, which investigated this hypothesis. Researchers measured oestrogen levels at different points of a female CrossFit athlete’s menstrual cycle and examined whether collagen supplementation had an effect on exercise-induced collagen synthesis (29)?
As expected, oestrogen levels were highest at the end of the follicular phase – shortly before ovulation. This resulted in a dampened exercise-induced collagen synthesis rate – measured by the blood marker PINP. However, ingesting 30g collagen prior to exercise reduced the negative effect of oestrogen on collagen synthesis.
PINP is only an indirect blood marker for collagen snythesis and gives no indication to what is actually happening in tendon or cartilage tissue
Further research is needed to elucidate whether such collagen intervention would actually translate to stronger tendons/healthier cartilage and potentially reduce risk of injuries.
How to take collagen:
Earlier findings suggested that about 10g of collagen peptides or gelatine would be the optimal dosage. However, more recent studies found 30g of collagen to be more effective in elevating plasma glycine and proline levels, as well as collagen synthesis rates – measured by PINP levels (30, 31).
Another study by Krims et al. (2024) used a different supplement protocol (32). Participants took 2 x 15g collagen, which did not affect connective tissue synthesis rate. It remains unclear whether the lack of observed effects is due to variations in dosage and timing.
Nevertheless, the overall evidence suggests that higher dosages – combined with ~50mg of Vitamin C – taken approximately 1-h before exercise, is the most effective protocol. Since training is necessary to activate collagen synthesis, taking collagen peptides on rest days is unlikely to provide any benefits.
Summary:
- Mechanistic in vitro studies indicate that collagen and gelatin may significantly enhance cartilage synthesis rates.
- Human studies seem to support these findings; however, they only measure collagen synthesis rates in the blood serum, which does not provide information about the effects on cartilage or ligament tissue.
- In individuals experiencing chronic joint pain, ongoing collagen supplementation for 8 weeks or longer may lead to a reduction in subjective pain levels by 15 to 20%.
- Overall, the evidence supporting the idea that collagen supplementation improves cartilage health is weak to moderate.
- Collagen may enhance tendon stiffness – but the quality of relevant studies is not high.
- Both whey and casein proteins do not appear to have an impact on cartilage tissue, even when consumed in higher quantities.
- More high-quality randomized controlled trials (RCTs) with human cartilage or tendon tissue are needed to draw definitive conclusions about the potential benefits of collagen supplementation on joint and tendon health.
Our assessement
Overall evidence for improving joint health

